Clinical trials look at new ways to prevent, detect or treat disease. By participating in a trial, you can help researchers discover new pathways and learn more about your personal health.
No matter what has made you curious or motivated you to join a clinical trial, education is critical so you understand the benefits, risks and processes.
WHAT ARE CLINICAL TRIALS?
Clinical trials are research studies that involve people. They’re the final step in a long process that begins with research in a lab and animal testing. Clinical trials aim to find out if a new medical treatment or strategy, like a drug, medical device, or procedure, is safe and effective for people.
They might also investigate ways to prevent diseases, diagnose conditions more accurately, or explore how to improve the quality of life for individuals with chronic illnesses. Clinical trials are essential in advancing medical knowledge and patient care.
WHO CAN JOIN A CLINICAL TRIAL?
Each clinical trial has specific eligibility criteria that determine who can participate. These criteria can include age, gender, type and stage of disease, previous treatment history, and other medical conditions. Some trials seek participants with specific illnesses or conditions, while others need healthy participants.
It’s important to remember that the criteria are not used to exclude potential participants personally, but rather to ensure the safety of the trial and to determine the effectiveness of the treatment.
TYPES AND PHASES OF TRIALS
There are several types of clinical trials, including treatment trials (testing new treatments), prevention trials (looking for better ways to prevent diseases), diagnostic trials (conducted to find better tests or procedures for diagnosing a particular disease), and quality-of-life trials (exploring ways to improve the quality of life for individuals with a chronic illness).
Clinical trials also go through various phases:
-
Phase 1 trials are the first stage of testing in humans and aim to identify side effects and determine the treatment’s safety. These typically involve a small number of participants.
-
Phase 2 trials continue to assess safety but also start to evaluate how well the new treatment works.
-
Phase 3 trials involve several hundreds to thousands of patients and provide the critical information about effectiveness and side effects required for regulatory approval.
-
Phase 4 trials happen after a treatment has been approved and are used to gather more information about long-term risks, benefits, and optimal use.
Remember, it’s important to discuss your options with your healthcare provider before deciding to participate in a clinical trial. They can provide personalized advice based on your situation and health status.
Use of placebos
Placebos are sometimes used in clinical trials to test whether the results of a drug are better than no treatment at all. In these cases, a placebo designed to look like the drug being tested, but without any of the active ingredients, is administered to some participants for the duration of the study.
If a placebo is used in a clinical trial, you’ll be informed before participating that patients will receive either the treatment being studied or the placebo, at random. Individual participants aren’t informed which one they’ll receive. If you have any questions about the effects of placebos or the reasons behind their use, ask your doctor or the clinical trial investigator.
WHAT ARE THE STEPS OF A CLINICAL TRIAL?
Participating in a clinical trial is a serious commitment with several stages that may require more time and attention than standard treatment. It can involve more frequent check-ups and medical tests, and a possibility of experiencing unknown side effects. It’s crucial for participants to communicate openly with the healthcare team, ask questions, and report any changes in health.
Screening: Initially, potential participants undergo screening processes to ensure they meet the trial’s eligibility criteria. These criteria can include factors like age, gender, type and stage of disease, previous treatment history, and other medical conditions. The screening process often involves tests and procedures, such as blood tests or imaging scans.
Informed Consent: Before participation, each individual must fully understand the nature of the trial, its purpose, the procedures involved, potential risks, and benefits. This is called the informed consent process. It ensures patients can make informed decisions about their participation. Patients have the right to withdraw from the study at any time, for any reason, without any impact on their standard medical care.
Treatment Phase: Once enrolled, participants will typically enter the treatment phase. This could involve testing new medical interventions, such as drugs or procedures, or comparing existing treatments. Participants might be grouped into different “arms” or segments of the study, with each receiving a different treatment.
Participants will receive regular medical care from a research team, which typically includes doctors, nurses, and other health professionals. They will monitor the participant’s health closely and provide specific instructions regarding the treatment.
Follow-Up: After the treatment phase, participants enter the follow-up phase. The research team continues to monitor the participants for specific periods to assess the long-term effects and efficacy of the treatment. This phase can last for months or even years.
Data Collection: Throughout the trial, researchers will collect data about the participant’s health, the effect of the intervention, and any side effects. This data is crucial for understanding the intervention’s effectiveness and safety.
Remember, each clinical trial is unique, just like each patient’s journey. The potential benefits, such as accessing new treatments and contributing to medical research, must be weighed against the potential risks. Always consult with your healthcare provider to make the best decision for your health.
I NEED SOME TERMINOLOGY HELP
When getting involved in a clinical trial – there can be a lot of new terminology you haven’t heard before. Below is some common terminology used during a clinical trial:
Abiraterone: A medication used to decrease the production of testosterone in the body, used in the treatment of prostate cancer.
Androgen Deprivation Therapy (ADT): Treatments that reduce levels of male hormones in the body to help slow the growth of prostate cancer.
Biomarkers: Biological molecules found in blood, other body fluids, or tissues that are a sign of a normal or abnormal process, or of a condition or disease.
Bosniak Classification: A system used to classify kidney cysts based on imaging characteristics on intravenous contrast-enhanced computed tomography (CT) scan.
ccRCC (Clear Cell Renal Cell Carcinoma): The most common type of kidney cancer.
CT (Computed Tomography): An imaging technique that uses a series of X-ray images to create cross-sectional images of the body.
Cytotoxic Chemotherapy: A type of chemotherapy that kills cells, especially cancer cells, whether they are at rest or dividing.
Darolutamide: A medication used to treat non-metastatic castration-resistant prostate cancer.
DDR Gene Mutated mCSPC (DNA Damage Repair Gene Mutated Metastatic Castration-Sensitive Prostate Cancer): Prostate cancer that has spread to other parts of the body, continues to respond to treatments to lower testosterone, and has mutations in the genes that help repair damage to DNA.
Enzalutamide: A medication used to treat prostate cancer.
Ex-vivo: Outside the living body, in a laboratory setting.
GAG Scores: These could potentially refer to a specific scoring or rating system used in the trial, but the exact meaning isn’t clear without more context.
Leibovich Points: A scoring system used to predict the risk of kidney cancer spreading after the kidney has been removed.
Leuprolide: A medication used to treat prostate cancer by reducing the level of testosterone in the body.
Luteinizing hormone releasing hormone analog (LHRHA): A synthetic hormone that helps to decrease testosterone levels, used in the treatment of prostate cancer.
mCRPC (Metastatic Castration-resistant Prostate Cancer): A stage of prostate cancer where the cancer has spread to other parts of the body and continues to grow despite treatments to lower testosterone.
Metastatic: Referring to cancer that has spread from its original location to other parts of the body.
mHSPC (Metastatic Hormone-Sensitive Prostate Cancer): A type of prostate cancer that has spread to other parts of the body and is still responding to treatments that lower testosterone levels.
New Hormonal Agents (NHAs): A newer class of medications that block the effect of androgens, used in the treatment of prostate cancer.
nmCRPC (Non-metastatic Castration-resistant Prostate Cancer): A stage of prostate cancer where the cancer has not spread beyond the prostate but continues to grow despite treatments to lower testosterone.
Non-steroidal anti androgen (NSAA): A type of medication that blocks the effects of androgens (male hormones) in the body, used in the treatment of prostate cancer.
Olaparib: A medication used in the treatment of certain types of cancer, including metastatic castration-resistant prostate cancer.
Overall Survival (OS): The length of time from either the date of diagnosis or the start of treatment for a disease, such as cancer, that patients diagnosed with the disease are still alive.
Pathological Complete Response (pCR): No evidence of cancer cells in a tissue sample removed during surgery or biopsy after treatment.
Pelvic Lymph Node Dissection (pLND): A surgical procedure to remove lymph nodes in the pelvic area to check for the spread of cancer.
Prospective Cohort: A research study that follows over time a group of similar individuals (cohorts) who differ with respect to certain factors under study, to determine how these factors affect rates of a certain outcome.
Proteomic: The large-scale study of proteins, particularly their structures and functions.
Radical Prostatectomy (RP): A surgical procedure to remove the entire prostate gland and some of the tissue around it, used to treat prostate cancer.
Radiographic Progression-Free Survival (rPFS): The length of time during and after treatment that a patient lives with cancer but it does not get worse as measured by imaging tests.
Survivorship: The health and life of a person following the end of cancer treatment.
Talazoparib: A medication used to treat certain types of cancer, including prostate cancer, in patients with specific genetic mutations.
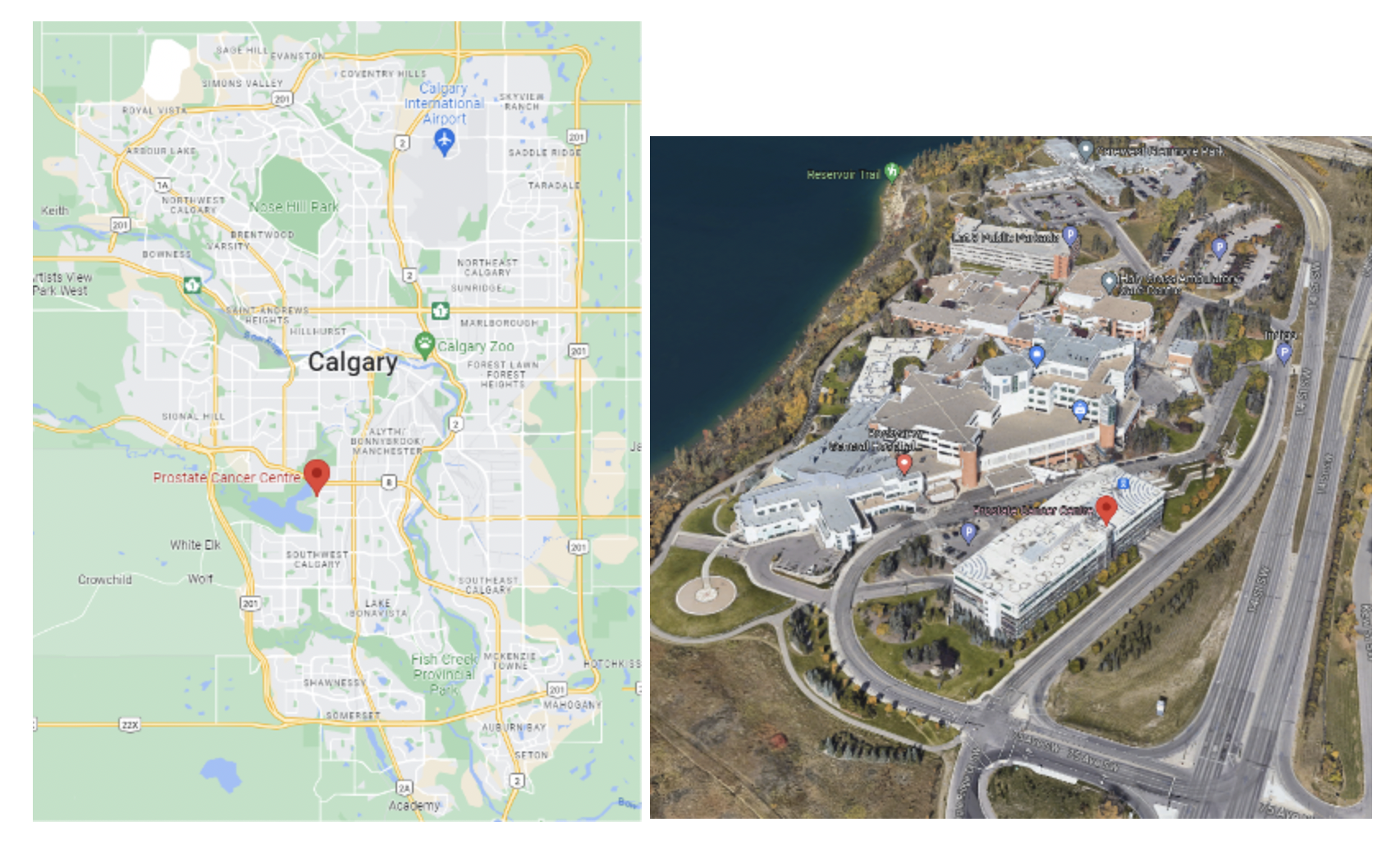
PLAN YOUR VISIT
Our goal is to keep you informed and supported at every step of your clinical trial experience. Keep reading to learn more about how to find us and what to expect during your first visit.
How to find us:
-
The Prostate Cancer Centre is located at 6500 7007 14 St SW Calgary, AB directly across the street from the Rockyview General Hospital.
-
If driving, park in Parking Lot 1 . The PCC is located on the 6th floor of the parking lot building. Parking can be challenging during peak hours so leave extra time.
-
Go to the elevator bank. There will be two elevators – one that goes to the main building and one that goes to the 6th This is noted on the elevator doors.
-
When you reach the 6th floor, there will be an information desk to help you with directions.
-
-
At reception, you’ll be welcomed by one of our Clinical Research Coordinators who will guide you through your visit.
-
Your privacy is paramount to us. All consultations take place in a private room.
-
Your visit will typically include specific procedures associated with your clinical trial. This may include:
-
Blood and/or vitals collection
-
Medical imaging procedures such as a CT scan, MRI, or bone scan
-
Receipt of the medication used for the trial.
-
Questionnaires about your medical history and quality of life
-
-
Depending on your visit’s schedule, you may or may not meet with the Urologist. However, all information gathered during your visit is reviewed by the Urologist.
-
Before you leave, we’ll schedule your next appointment.